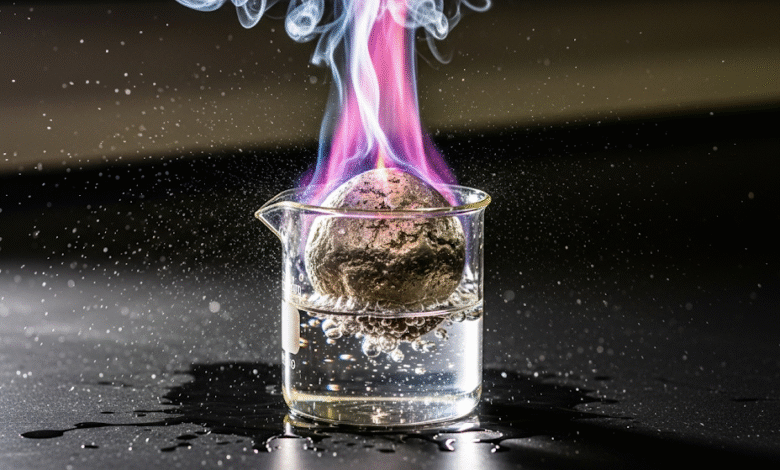
The most electropositive elements are the alkali metals and alkaline earth metals. These elements have a very low ionization energy and a high affinity for losing electrons.

What is Electropositivity?
Electropositivity is the opposite of electronegativity. It’s a measure of an element’s ability to donate electrons and form positive ions. Think of it as how “willing” an atom is to give away its electrons. The more easily an element gives away its electrons, the more electropositive it is.
The Most Electropositive Elements
The most electropositive elements are located on the far left side of the periodic table. They are the alkali metals (Group 1) and the alkaline earth metals (Group 2).
Group 1: Alkali Metals
These include lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr). They have only one valence electron, which they donate to achieve a stable electron configuration. As you go down the group, the atoms get larger, and the single valence electron is further from the nucleus, making it even easier to remove. Therefore, cesium (Cs) and francium (Fr) are the most electropositive elements in this group.
Group 2: Alkaline Earth Metals
These include beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra). They have two valence electrons. While still very willing to donate electrons, they are slightly less electropositive than the alkali metals because of their two valence electrons and a slightly higher effective nuclear charge.
Why are they so Electropositive?
The electropositivity of an element is related to two main factors:
- Atomic Size: As the size of an atom increases, the outermost electrons are farther from the nucleus. This weakens the attraction between the nucleus and these electrons, making them easier to remove.
- Nuclear Charge: The less the positive charge of the nucleus “pulls” on the outer electrons, the easier they are to give up.
Since the alkali metals and alkaline earth metals have large atomic radii and relatively low nuclear charges for their size, they readily lose their outermost electrons, making them highly electropositive.



